Overview
Fire prevention refers to reducing the flammability of materials in flames and slowing down the spread of flames. When the flame is removed, it can self-extinguish quickly and no longer smolder.
1. Combustion process and conditions
The combustion process of fiber materials, that is, from ignition to final combustion products, requires a series of complex physical and chemical changes. These changes have obvious stages and can usually be Divided into:
(1) Thermal cracking of materials produces flammable gases, non-flammable gases and carbonized residues.
(2) Combustible cracked gas and oxygen are mixed. When the temperature reaches the ignition point or encounters other fire sources, it ignites and releases heat, smoke and smoke.
(3) The heat released causes the fiber to continue to crack and burn, causing the flame to spread.
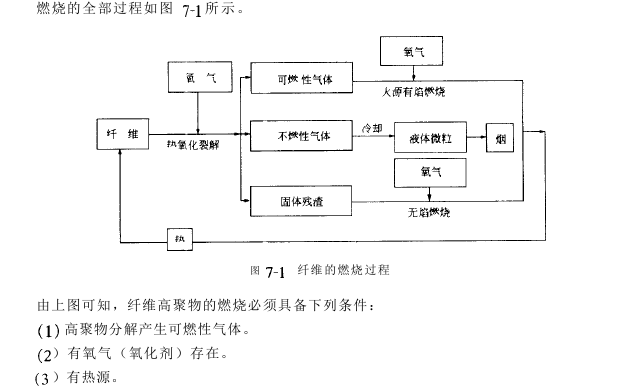
To continue burning after the burned fiber polymer material leaves the fire source, the following conditions must be met.
(1) The heat energy generated by combustion is enough to heat the polymer, causing it to continuously produce flammable gas.
(2) The generated flammable gas is mixed with oxygen and spreads to the ignited part, or the burning part spreads to the mixed area of flammable gas and oxygen.
In this way, the three elements of combustibles, heat and oxygen constitute the combustion cycle.
In addition to the gas-phase flaming combustion of the above-mentioned flammable gases and oxygen, there is also the solid-phase flameless combustion (also called smoldering) of the carbonized residue formed during cracking and oxygen. The temperature required for flameless combustion is much higher than that of flaming combustion, but smoldering can also burn materials, and sometimes the flame suddenly breaks out into flaming combustion and causes a fire.
2. Combustion system
The spatial distribution of the combustion process can generally be divided into 5 zones: condensed phase heating zone; condensed phase reaction zone; gas phase heating reaction zone; flame zone; combustion product zone. Different physical and chemical changes occur in each area.
In the condensed phase heating zone, the temperature of the polymer gradually increases under the action of the heat source, and physical heat absorption occurs.
In the condensed phase reaction zone, due to the effect of high temperature, chemical reactions such as thermal cracking, thermal oxidative cracking, dehydrogenation, condensation, cyclization and carbonization will further occur depending on the polymer structure and heating conditions. Some of these reactions are exothermic and some are endothermic.
Various cracking products and a small amount of dispersed particulate carbon generated in the condensed phase reaction zone are continued to be heated during the diffusion process to the gas phase heating reaction zone, causing further cracking, and mixed with oxygen in the air to react and release heat. In addition to being used for cracking in this zone, some of this heat is also fed back to the condensed phase reaction zone to maintain the progress of the reaction in the condensed phase reaction zone. Because the gas phase heating reaction zone is close to the surface of the material, the temperature is not too high, and the concentration of the cracking products is relatively large. , the oxygen concentration is small, therefore, incomplete combustion occurs and some particulate carbon with a similar structure to graphite is formed, or a carbonized layer is formed on the surface of the material.
When the deep cracking products and oxidation products escaping from the gas-phase heating reaction zone diffuse to the flame zone, all combustion reactions are completed due to the high oxygen concentration in the flame zone. The heat released by the reaction is either transferred to the adjacent gas phase heating reaction zone, or transferred to other zones in the form of radiation. Obviously, the ratio of feedback energy to energy diffused to the outside is related to the shape of the burning object and the way the flame spreads.
It is a continuous process, and there are no absolute boundaries between regions. They combine with each other to form combustion. In fact, combustion is a continuous burning wave.
From the above analysis, it can be seen that during steady-state combustion, the physical and chemical changes that occur in each area need to be maintained by the heat released by its own reaction or the heat transferred into the high-temperature area. The reactants in each area are maintained by their own reaction. The reaction itself occurs, or enters from the low temperature area. Among them, the reaction occurring in the condensed phase reaction zone has a great influence on gas phase combustion. The combustible gas generated by the cracking reaction is the raw material of the gas phase combustion reaction and can promote the spread of flames. However, the coking layer formed by deoxidation and carbonization can inhibit the transfer of heat to the condensed phase heating zone and condensed phase reaction zone due to its good thermal insulation properties, thus causing Produce certain fire protection effect.
3. Fire prevention mechanism and method
From the analysis of combustion process, combustion conditions and combustion system, it can be seen that in order to achieve the purpose of fire prevention, it is necessary to cut off the combustion cycle composed of three elements: combustibles, heat and oxygen. The function of fire retardant is to inhibit or terminate the three elements of fiber burning in some way to achieve the purpose of fire prevention.
It is generally believed that the fire protection effect of brominated fire retardants is mainly carried out in the gas phase. The main reason is that brominated fire retardants decompose into HBR when heated, and HBR can capture and transfer active free radicals (HO·, O·, H·), generate less active bromine radicals to reduce the gas phase combustion speed and slow down or terminate the burning of fibers.



AAADFGTEHTRY