42. Can chelating agents replace water softeners?
Answer Chelating agent, also known as complexing agent, is an organic or inorganic compound that can chelate with heavy metal ions to form a stable water-soluble complex, thereby passivating heavy metal ions. The molecule of this compound contains electron donors that can coordinate with heavy metal ions, so it has a series of extraordinary functions such as water softening, detergency, rust prevention, stabilization, and efficiency enhancement. Common chelating agents in printing and dyeing processes include the following. (1) Phosphates. Mainly include sodium tripolyphosphate, sodium polyphosphate, sodium hexametaphosphate, sodium pyrophosphate, etc. This type of chelating agent was an early water softener used in the printing and dyeing industry due to its ion exchange ability. Sodium pyrophosphate can form complex ions with ferric ions, so it can be used in hydrogen peroxide stabilizers. But inorganic phosphates have been banned in some areas. (2) Aminocarboxylic acids. The main ones are ethylenediaminetetraacetic acid (EDTA), which is water softener B; nitrilotriacetic acid (NTA), which is water softener A. In addition, there are secondary ethyl triamine pentaacetic acid (DTPA), N-hydroxyethyl ethylamine triacetic acid (HEDTA), ethylene glycol-bis-(β-aminoethyl ether)-N, N’-tetraacetic acid (ECTA) )wait. The ligands of aminocarboxylic acid chelating agents are nitrogen atoms and negatively charged carboxylate ions (-COO-). The greater the number of ligands, the stronger the complexation with metal ions. Among them, DTPA has strong complexing effect with most metal ions, followed by EDTA and HEDTA, and NTA is poor. Among them, DTPA is effective as a hydrogen peroxide stabilizer. However, NTA, EDTA, DTPA, etc. have extremely poor biodegradability after chelating metals, and their use has been strictly prohibited in some European countries in recent years. (3) Organic phosphonic acids. Mainly include aminotrimethylenephosphonic acid (ATMP), 1-hydroxyethylene-1, 1-diphosphonic acid (HEDP), ethylenediaminetetramethylenephosphonic acid (EDTMP), diethylenetriaminepentamethylene phosphonic acid (DTPMP), ammonium trimethylene phosphonic acid (ATP), etc. This type of chelating agent has the ability to disperse and suspend dirt and is not easily hydrolyzed at high temperatures. It has an excellent effect on preventing pot scale and can also be used as a boiler cleaning agent. DTPMP is a more effective hydrogen peroxide stabilizer than DTPA. DTPA only has a better stabilizing effect on Ca and Mg salts in the presence of sodium silicate, while DTPMP can also stabilize hydrogen peroxide without adding sodium silicate. Play a stabilizing role. This type of chelating agent not only has good chelating and descaling effects, but is also easily biodegradable, and is currently used more frequently. (4) Hydroxycarboxylic acids. Mainly include gluconic acid, polyacrylic acid (PAA), maleic acid (MAO), etc. The water softening ability of this type of chelating agent generally depends on the segment structure of the polymer and the number of carboxyl groups and substituents. They not only have chelating ability, but also dispersing ability. The important properties of this type of chelating agent are good biodegradability and compliance with Environmental protection requirements. It can be seen from the types of chelating agents mentioned above that water softener A and water softener 8 are one of the varieties of aminocarboxylic acid chelating agents. Therefore, this type of chelating agent can replace the water softener. However, among water softeners, such as sodium hexametaphosphate, water softener A and water softener 8, there are certain differences in their properties and water softening effects. The water softening effect of water softeners such as sodium hexametaphosphate is only ion exchange. Water softeners A and B can form complexes with metal ions. Water softener A only combines with calcium and magnesium ions contained in hard water to form a water-soluble complex, thereby achieving the effect of softening water, while water softener 8 can form stable complexes with many metal ions (except alkali metals) , usually can form complexes with divalent calcium and magnesium ions, which are very stable in acidic or alkaline solutions, and can form complexes with trivalent metal ions such as Fe3+, etc. Stable in solutions with a pH value of 1~2. Complexes formed with tetravalent metal ions such as Ti4+, or even 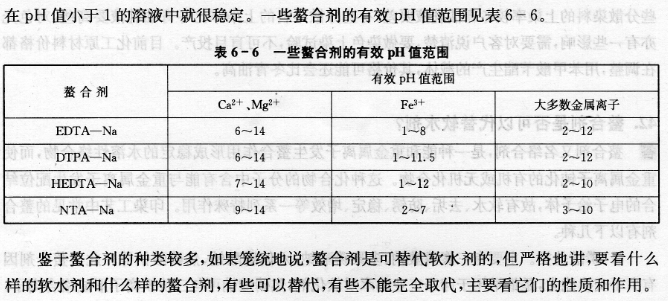
AAADBGRTHRTH
Disclaimer:
Disclaimer: Some of the texts, pictures, audios, and videos of some articles published on this site are from the Internet and do not represent the views of this site. The copyrights belong to the original authors. If you find that the information reproduced on this website infringes upon your rights, please contact us and we will change or delete it as soon as possible.
AA