In the pharmaceutical production process, especially for non-terminal sterile products, bacterial filtration and aseptic production processes are mainly relied on to achieve sterility control requirements. With the widespread application of bacterial filtration technology in the pharmaceutical industry, the risks of bacterial filtration have also attracted major attention in the industry. In order to strengthen the supervision of drug production, standardize the scientific and systematic development of Bacterial Filtration Membrane technology and application by drug manufacturers, and ensure the reliability, effectiveness and quality stability of sterile drugs, the State Food and Drug Administration organized and formulated the “Filtration Membrane” “Technical and Application Guide”, as a guiding document for the implementation of “Good Manufacturing Practice for Drugs (2010 Revision)”, will be effective from October 1, 2018.

Because biopharmaceuticals are produced under sterile conditions, environmental controls and robust and qualified aseptic processes are required to Ensure sterility.
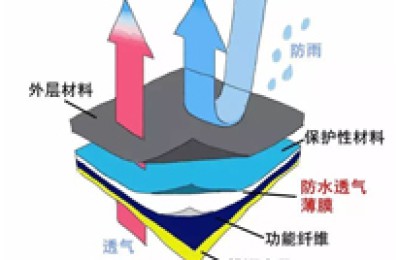
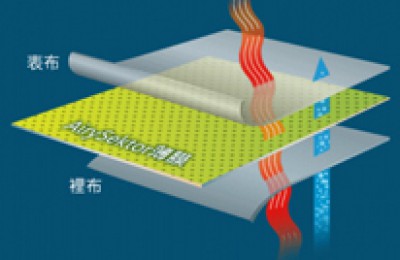
At present, the PTFE bubble point membrane series independently developed by Bacterial Filtration Membrane manufacturers is widely used in organic solvent filtration. It has hydrophobicity and Two categories of hydrophilicity. It can achieve sterility when used for gas filtration, has the characteristics of large flux, temperature resistance, resistance to strong acids and alkalis, and wide chemical applicability. It is suitable for fermentation tanks, carbon dioxide, nitrogen and compressed air. When used for liquid filtration, it can intercept all kinds of phages, small bacteria and particles above 0.2μm. The bacterial filtration membrane meets the requirements of HIMA small bacteria interception experiments.
It is a bacterial filtration membrane environmental protection material manufacturer that specializes in R&D, production and sales. Our company is different from other manufacturers. Our company can according to the user’s requirements. By optimizing the combination of key technical parameters such as speed ratio, pressure ratio, and operating temperature, the air permeability, thickness, and void ratio can be adjusted online according to the ratio and pore-forming medium to meet your different requirements. Interested parties can enter the store for consultation.
</p