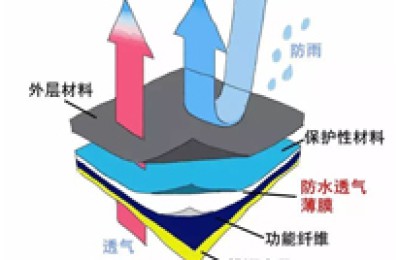
The color fastness test to rubbing refers to A test in which the colored samples are rubbed with dry rubbing cloth and wet rubbing cloth respectively, and then the degree of staining of the rubbing cloth is evaluated. The test results are divided into 5 levels, with level 5 being the best and level 1 being the worst. Although the testing process is simple, it is the most basic color fastness assessment indicator for textile products. It is almost one of the items that buyers from all over the world must assess when placing orders. The technical conditions of rubbing fastness testing standards in various countries are very similar, but there are also some differences. —[Textile]
During the friction process between textiles and other objects, the color shedding or the degree of staining of the rubbed objects is affected by many factors. There are two ways for color to fall off and stain: First, the dye on the textile falls off or fades and is stained on the surface of the friction object;
First, the dyed fibers fall off and adhere to the friction Object surface.
Although they have different chemical structures There are certain differences in the covalent bond strength and adhesion between reactive dyes and cellulose fibers, but their effects on the wet rubbing fastness of dyed fabrics are basically the same. —[600D Oxford cloth]
When dyed fabrics are wet rubbed, the covalent bonds formed between the dye and the fibers will not break and cause floating colors. The transferred dye usually does not form a covalent bond with the fiber, but only relies on van der Waals forces to produce adsorption, that is, floating color.
Light fastness The test refers to placing a textile sample and a set of blue wool standard samples under artificial light sources for exposure under specified conditions, and then comparing the discoloration of the two to evaluate the color fastness.
Improvement Pathways to light fastness: The photofading mechanism of dyes is very complex, but the main reason is that the dye is excited after absorbing photons, and a series of photochemical reactions occur to destroy the structure, leading to discoloration and fading. The light fastness of textiles mainly depends on the chemical structure of the dye, as well as its aggregation state, combination state and mixed color matching. Therefore, the rational selection of dyes is very important.
For cellulose fibers For textiles, dyes with better oxidation resistance should be selected; for protein fibers, dyes with better resistance to reduction or containing weak oxidizing additives should be used; for other fibers, dyes should be selected based on their impact on fading. In order to enhance the photooxidation stability of the azo group in the dye molecular structure, during the dye synthesis process, some strong electron-withdrawing groups are usually introduced at the ortho-position of the azo group, thereby reducing the electron cloud density of the azo gas atom. —[300D Oxford cloth]
In addition, hydroxyl groups can also be introduced at the two ortho-positions of the azo group, and their coordination ability can be used to complex with heavy metals, thereby reducing the electron cloud density of the hydrogen atoms of the azo group and coupling The nitrogen group acts as a shield, ultimately improving the light fastness of the dye.
Suzhou Textile Co., Ltd. :www.wcxfz.com
</p